Improving heart disease prediction and prognosis
How simulating arterial flow might lead to decreased heart disease mortality
Coronary heart disease (CHD) resulting from plaque build-up in the arteries (atherosclerosis) remains the most common cause of hospital admissions and mortality in many developed countries. It is thought that arterial geometry, cell function and blood-flow dynamics play an important role in triggering CHD. Wall shear stress and disturbed flow in the vicinity of wide angle bifurcations have also been pinpointed as potential triggers of arterial plaque build-up.
In collaboration with NeSI and the University of Canterbury’s High Performance Computing department (UC HPC), PhD student Stewart Dowding, Dr Constantine Zakkaroff and Prof. Tim David have developed the Coupled Arterial Cells project. At its core, this project has a massively parallel, multi-scale software framework for modelling cellular-level signalling with the goal of providing invaluable insight into coronary heart disease. Computer (or in silico) simulations enable the rapid exploration and pruning of the parameter search space for the refinement and integration of micro-level models into biologically realistic macro-scale models. This project allows researchers to obtain large-scale emergent behaviour data that can’t be harvested from in vivo or in vitro experiments. More generally, the coupled cells simulations provide the opportunity to perform physiological experiments otherwise impossible to conduct in a clinical environment.
Atherosclerotic plaque lesions have characteristic length scales much larger than a single arterial cell, hence the mechanisms of plaque formation must be studied over considerable distances, typically hundreds to thousands of cell lengths. Current simulations include approximately 1.2 million coupled arterial cells whereas a complete coronary tree might contain on the order of 100 million arterial cells. One of the milestones of this project is to increase the simulation size in order to get a full picture of the emergent macro-scale behaviour of plaque deposition at the level of a complete coronary tree.
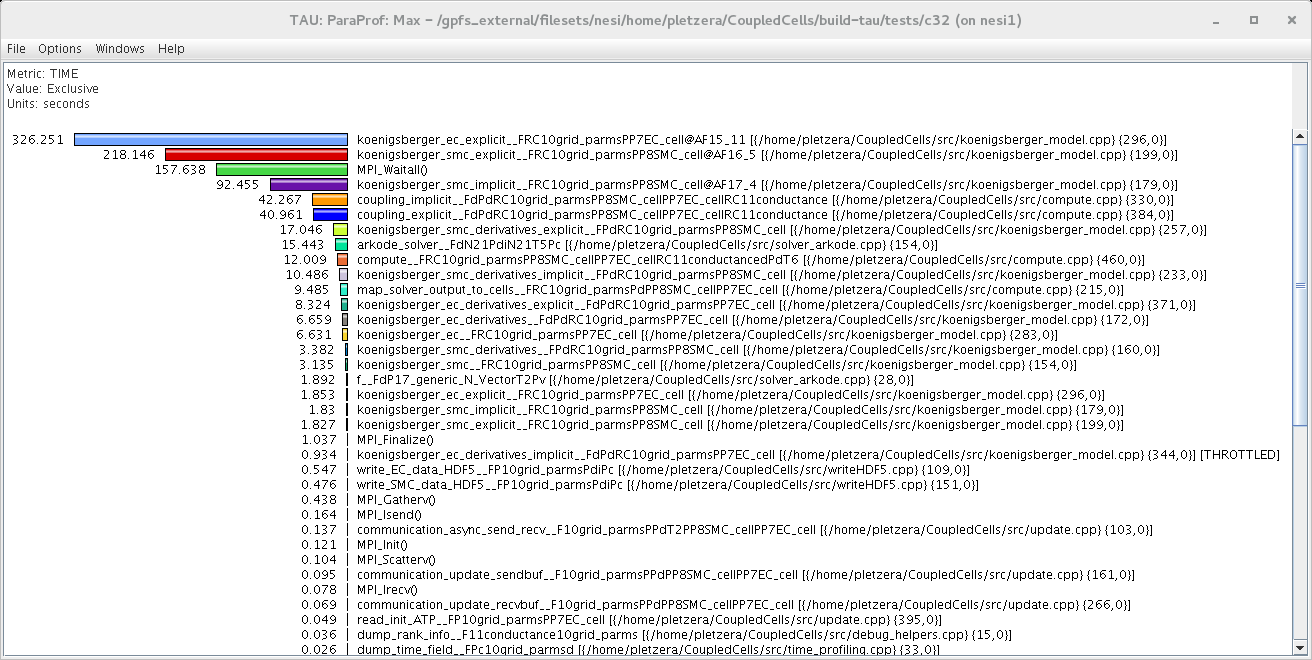
NeSI was called in to help port the Coupled Arterial Cells project from UC HPC’s BlueGene platform to Fitzroy, the supercomputer operated by NIWA for national weather forecasting. In the process, NeSI improved the CMake build system by adding unit tests and the option to instrument the code with TAU (Tuning and Analysis Utilities). Profiling showed that most of the CPU time (60%) was spent in two procedures (see below). Further investigations revealed that these procedures were already highly optimised and, to gain further performance improvements, one would need to introduce thread based OpenMP parallelisation at the level of loops. The team has now embarked on generalising the domain decomposition in the code to allow for more cells per sub-domain. This will make the Coupled Arterial Cells code ideally suited to run on massively parallel architectures with many cores per node, such as Fitzroy and future NeSI platforms.
Our small-segment arterial simulation results show that wide bifurcation angles can suppress arterial wall calcium signalling. Figures 2 and 3 below show two snapshots of coupled arterial cells simulations for 60 and 100 degree bifurcation angles. These results demonstrate that wider-angle bifurcations are likely to interfere with calcium dynamics in smooth muscle cells which is known to lead to plaque formation.
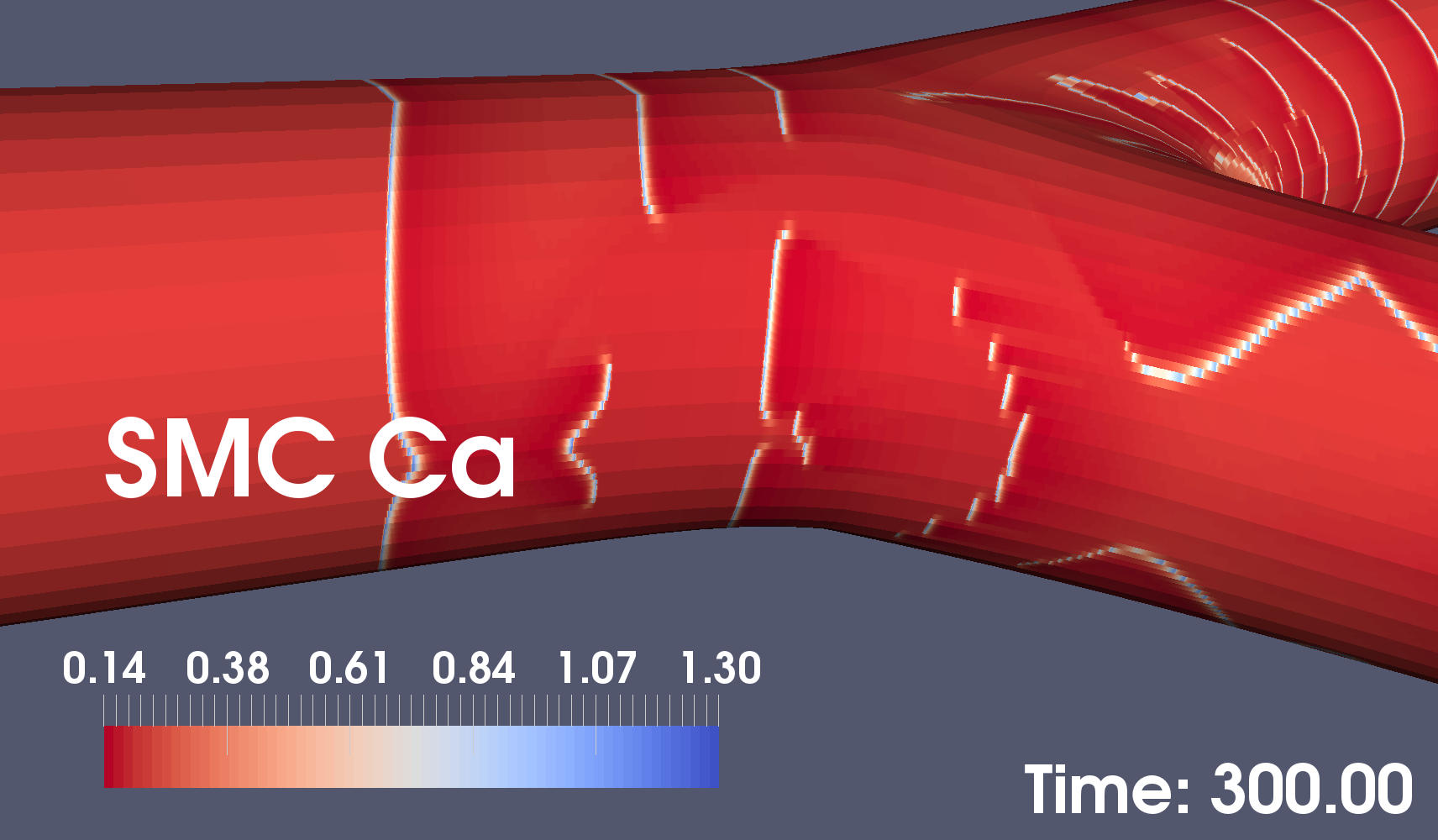
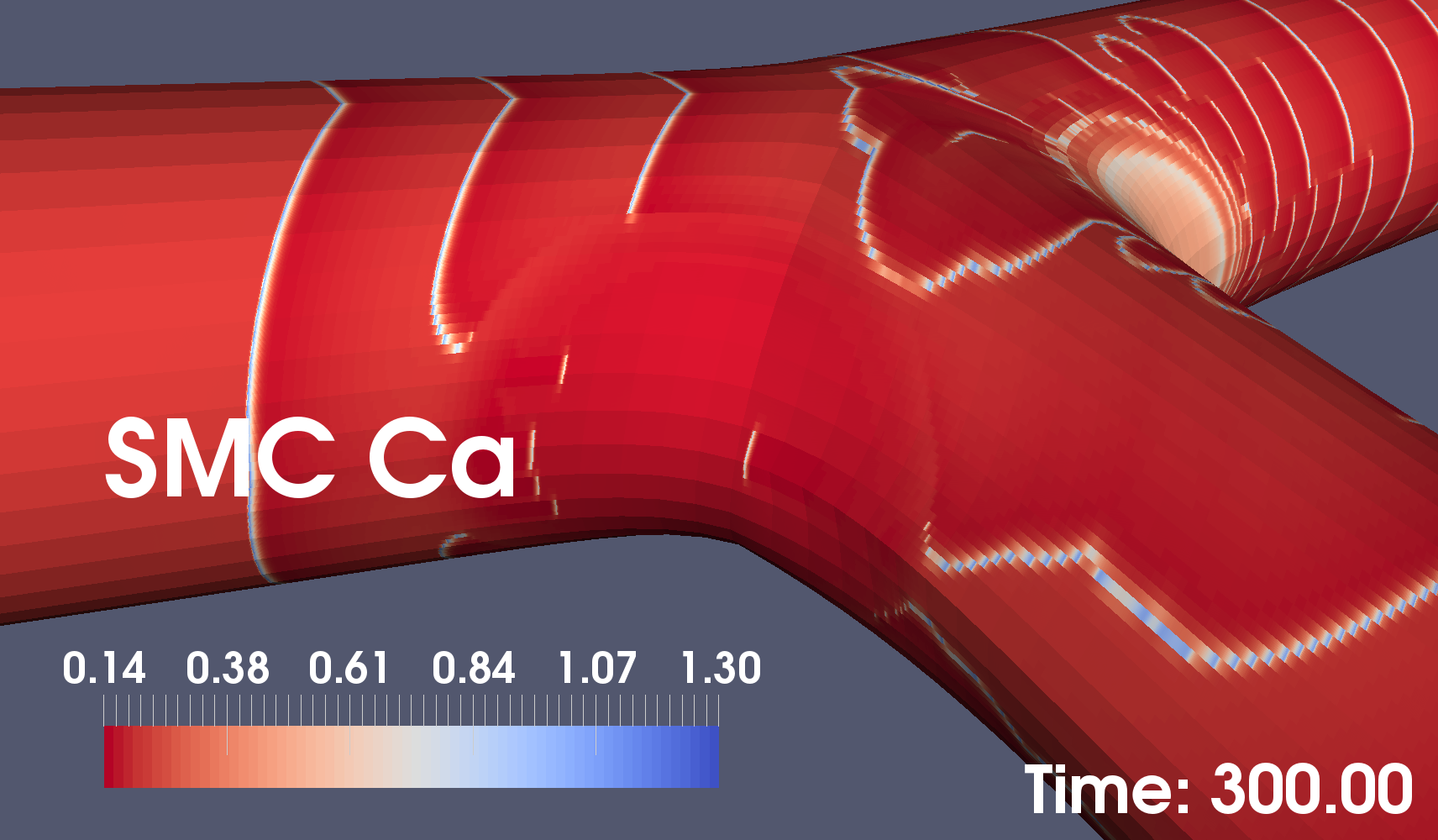
In the lower figure, the near absence of calcium waves in the area closest to the camera may lead to calcium plaques.
You can see the above images in motion on the UC HPC Research YouTube Channel:






